News
Functional Shapes (FShapes) for Morphometric Analysis of Osteoarthritis: Collaboration with Center for Imaging Science Yields New Insight
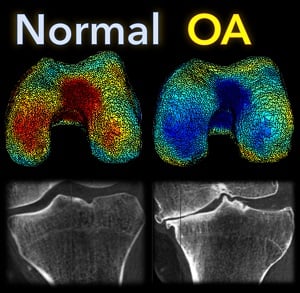 A new paper by Nicolas Charon (Assistant Professor, Center for Imaging Sciences and Applied Mathematics and Statistics at Johns Hopkins University), Asef Islam (undergraduate student in Biomedical Engineering at Johns Hopkins), and Wojtek Zbijewski (Assistant Professor in Biomedical Engineering at Johns Hopkins) investigates the feasibility of using the recently introduced framework of functional shapes (FShapes) to reveal morphological features of knee osteoarthritis (OA).
A new paper by Nicolas Charon (Assistant Professor, Center for Imaging Sciences and Applied Mathematics and Statistics at Johns Hopkins University), Asef Islam (undergraduate student in Biomedical Engineering at Johns Hopkins), and Wojtek Zbijewski (Assistant Professor in Biomedical Engineering at Johns Hopkins) investigates the feasibility of using the recently introduced framework of functional shapes (FShapes) to reveal morphological features of knee osteoarthritis (OA).
The concept of FShapes is promising for applications in OA because it provides a rigorous mathematical framework to simultaneously model the population variability in bone shape – here, the tibial or femoral articular surface – and in a function defined on that shape – here, a map of local joint space width at each point of the surface. Considering that articular degeneration is the hallmark of advanced OA, this approach might, for example, yield new insights into interactions between certain morphological bone variants and development of joint space loss.
The study used a set of three-dimensional knee scans of patients with and without OA. The scans were obtained using a novel weight-bearing extremity Cone Beam CT (CBCT) system at Johns Hopkins. After extracting tibial surface meshes from the CBCT images, each surface was equipped with a joint space map (JSM) using an algorithm based on an electrostatic model of the intra-articular space (previously developed at I-STAR). An atlas estimation procedure in the setting of large diffeomorphic deformation metric mapping (LDDMM) was then applied to obtain a template representing a mean shape and a mean JSM, together with variables that model the shape and JSM transformations from the template to each subject in the dataset. Therein lies another potential advantage of this approach: it is landmark-free because the diffeomorphic transformation model does not require a priori point correspondences between the subjects.
In a preliminary validation study, a support vector machine classifier was applied to the template-subject transformations to find discriminative features associated with OA. The correct classification scores were 85% – 91%, depending on which components of the FShape (shape+JSM, only shape, only JSM) were used. The discriminant directions revealed by this analysis were consistent with prior studies of OA: including medial joint space loss and deepening of the tibial plateau.
The functional shape methodology is a promising new tool for landmark-free morphological analysis in OA and other orthopedic applications where bone shape and alignment are simultaneously involved, ranging from joint disease to fracture healing.
(Link to paper) Nicolas Charon, Asef Islam, and Wojciech Zbijewski, “Landmark-free morphometric analysis of knee osteoarthritis using joint statistical models of bone shape and articular space variability” J. of Medical Imaging, 8(4), 044001 (2021)
SPIE Medical Imaging 2021 – Digital Forum – Presentations from the I-STAR Lab
 The SPIE Medical Imaging 2021 symposium has gone digital– a Digital Forum combining live events with pre-recorded presentations and communication via slack.
The SPIE Medical Imaging 2021 symposium has gone digital– a Digital Forum combining live events with pre-recorded presentations and communication via slack.
Presentations from the QuantIS Lab at SPIE 2021 Digital forum include abstracts and presentations at the following links for both the Physics of Medical Imaging and Digital Pathology and Computational Pathology conference::
PHYSICS OF MEDICAL IMAGING:
Liu et al., Quantitative dual-energy imaging in the presence of metal implants using locally constrained model-based decomposition, SPIE Physics of Medical Imaging (LINK).
Zhao et al., Image-domain cardiac motion compensation in multidirectional digital chest tomosynthesis, SPIE Physics of Medical Imaging (LINK).
Zhao et al., Effects of x-ray scatter in quantitative dual-energy imaging using dual-layer flat panel detectors, SPIE Physics of Medical Imaging (LINK).
DIGITAL AND COMPUTATIONAL PATHOLOGY
Poinapen et al. Three-dimensional shape and topology analysis of tissue-cleared tumor samples, SPIE Digital and Computational Pathology (LINK).
Congratulations to Danny Poinapen on the Best Poster Award in the Digital Pathology conference!
3D Image Reconstruction with “Known Components” Improves Dual-Energy Cone-Beam CT – New Paper by Stephen Liu et al.
Dual-energy (DE) decomposition has been adopted in orthopedic imaging to measure bone composition and visualize intraarticular contrast enhancement. One of the potential applications involves monitoring of callus mineralization for longitudinal assessment of fracture healing. However, fracture repair usually involves internal fixation hardware that can generate significant artifacts in reconstructed images.
 A paper by Stephen Liu at the I-STAR Lab at Hopkins BME addresses this challenge. The authors have developed a novel approach that augments their previous model-based material decomposition algorithm (MBMD) with the Known-Component (KC) reconstruction framework. Compared to conventional approaches, MBMD enables direct projection-based decomposition from systems where the two energy channels are acquired using non-coinciding rays. To mitigate metal artifacts in MBMD, the KC framework incorporates a digital model of the surgical hardware to inform the decomposition about the location and attenuation properties of the metal components.
A paper by Stephen Liu at the I-STAR Lab at Hopkins BME addresses this challenge. The authors have developed a novel approach that augments their previous model-based material decomposition algorithm (MBMD) with the Known-Component (KC) reconstruction framework. Compared to conventional approaches, MBMD enables direct projection-based decomposition from systems where the two energy channels are acquired using non-coinciding rays. To mitigate metal artifacts in MBMD, the KC framework incorporates a digital model of the surgical hardware to inform the decomposition about the location and attenuation properties of the metal components.
The algorithm was applied to simulated DE data representative of a dedicated extremity cone-beam CT (CBCT) employing an x-ray unit with three vertically arranged sources. This system is an attractive platform for fracture follow-up because it enables weight-bearing
3D imaging to assess the stability of the healing bone. The scanner generates DE data with non-coinciding high- and low-energy projection rays when the central source is operated at high tube potential and the peripheral sources at low potential. The algorithm was validated using a digital extremity phantom containing varying concentrations of Ca-water mixtures and Ti implants. Decomposition accuracy was compared to MBMD without the KC model.
The method suppressed metal artifacts and yielded estimated Ca concentrations that approached the reconstructions of an implant-free phantom for most mixture regions. In the vicinity of simple components, the errors of Ca density estimates obtained by incorporating KC in MBMD were ~1.5 – 5x lower than the errors of conventional MBMD; for cases with complex implants, the errors were ~3 – 5x lower.
In conclusion, the proposed method can achieve accurate bone mineral density measurements in the presence of metal implants using non-coinciding DE projections acquired on a multisource CBCT suitable for weight-bearing assessment of fractures.
Stephen Z Liu, Qian Cao, Matthew Tivnan, Steven W Tilley II, J Webster Stayman, Wojciech Zbijewski, Jeffrey H Siewerdsen Model-based dual-energy tomographic image reconstruction of objects containing known metal components. Phys Med Biol. 2020 Oct 28. doi: 10.1088/1361-6560/abc5a9.
New Multi-Institutional Project with Dr. Zbijewski on Forensic Analysis of Bone Morphology using CT
 Dr. Wojciech Zbijewski (Assistant Professor in Biomedical Engineering and PI in the I-STAR Lab) is among the Co-Principal Investigators in a new multi-institutional project that uses high-resolution CT and statistical modeling of bone shape and microstructure for forensic analysis. Sponsored by the U.S. Department of Justice, the new project tackles the challenge of estimating body mass and/or BMI in the skeletal remains of unidentified individuals.
Dr. Wojciech Zbijewski (Assistant Professor in Biomedical Engineering and PI in the I-STAR Lab) is among the Co-Principal Investigators in a new multi-institutional project that uses high-resolution CT and statistical modeling of bone shape and microstructure for forensic analysis. Sponsored by the U.S. Department of Justice, the new project tackles the challenge of estimating body mass and/or BMI in the skeletal remains of unidentified individuals.
Three research groups are involved in the effort: Dr. Zbijewski and team at Johns Hopkins Biomedical Engineering to work on CT imaging and shape and texture analysis; Dr. Adam Sylvester at the Johns Hopkins Center for Functional Anatomy and Evolution, an expert in statistical modeling of skeletal morphology; and Dr. Daniel Wescott and Dr. Deborah Cunnigham at Texas State University, the project lead site with resources and technical expertise related to large repositories of skeletal samples.
In identifying individuals based on skeletal remains, forensic anthropologists are faced with numerous challenges, including the need to estimate body mass and/or BMI. Particularly as the prevalence of obesity increases, the ability to assess BMI from skeletal remains could be of major benefit in establishing a biological profile in medicolegal death investigations.
The research aims to develop a reliable means of estimating BMI using CT images of skeletal remains combined with statistical shape and texture analysis. The analysis includes quantitative imaging of joint size, trabecular bone structure, diaphyseal cross-sectional properties, and whole-bone shape properties and establishes reliable correspondence between such features and body mass and/or BMI. By combining macro-scale shape analysis with micro-scale texture analysis, the researchers aim to establish whether such correspondences exist and the accuracy with which BMI can be accurately determined from skeletal remains. Preliminary studies suggest that the link may be evident in weight-bearing skeletal elements, such as the calcaneus, talus, tibia, femur, and the 4th lumbar vertebra. The project aims to establish reliable CT-based markers of BMI and translate the research findings into a software package for law enforcement to facilitate forensic analysis of skeletal remains.
Congratulations to Dr. Zbijewski and team on this exciting new project.
